Fluoride contamination in drinking water remains a widespread public health concern, particularly in arid and semi-arid regions where groundwater is the primary source of potable water. Chronic exposure to elevated fluoride levels—commonly above the World Health Organization’s (WHO) recommended limit of 1.5 mg/L—can result in dental and skeletal fluorosis, affecting millions of people globally. Affordable and effective defluoridation technologies are urgently needed, especially in low-income rural settings. In this study, the fluoride removal efficiency of calcium-spiked and non-spiked Moringa oleifera seed powder was investigated through controlled laboratory batch adsorption experiments. Biosorbents were prepared by treating ground seed powder with 1% calcium chloride solution and characterised based on their performance across five fluoride concentrations (1-20 ppm). Key parameters such as removal efficiency, residual fluoride levels, and adsorption capacity (qe) were evaluated under consistent operating conditions (pH 7, 2 g/50 mL dose, mesh 40, 120 minutes). Results indicated that calcium-spiked Moringa oleifera powder significantly outperformed its non-spiked counterpart. At 1 ppm, the spiked adsorbent achieved 94.35 ± 1.15% removal efficiency, compared to 81.45 ± 1.35% for the non-spiked. At the highest tested concentration (20 ppm), the spiked biosorbent still removed 72.31 ± 1.80% of fluoride, while the non-spiked removed only 54.21 ± 1.95%. Linear regression models showed strong inverse correlations between fluoride concentration and removal efficiency (R2 > 0.99, p < 0.001). The spiked adsorbent also resulted in significantly lower residual fluoride concentrations, with final values closer to the WHO guideline. One-way ANOVA confirmed significant differences in adsorption capacity and efficiency between treatments (p < 0.001). These findings highlight the effectiveness of calcium modification in enhancing biosorption performance and suggest that calcium-spiked Moringa oleifera seed powder is a promising, low-cost, and environmentally friendly solution for mitigating fluoride contamination in drinking water.
| Published in | American Journal of Physical Chemistry (Volume 14, Issue 4) |
| DOI | 10.11648/j.ajpc.20251404.11 |
| Page(s) | 91-99 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2025. Published by Science Publishing Group |
Fluoride Removal Efficiency, Moringa Oleifera biosorbent, Calcium Spiked Moringa Seeds, Adsorption Efficiency of Moringa Oleifera, Defluoridation, Residual Fluoride Ions, Drinking Water Safety
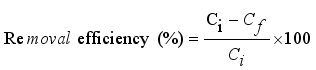
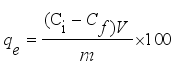
Initial Fluoride Concentration (ppm) | Fluoride Removed per Gram (mg/g) | Std. Dev (mg/g) |
|---|---|---|
1 | 0.47 | ± 0.01 |
1.5 | 1.06 | ± 0.05 |
5 | 4.43 | ± 0.10 |
10 | 8.85 | ± 0.15 |
20 | 42.35 | ± 0.50 |
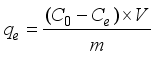
ANOVA | Analysis of Variance |
CaCl2 | Calcium Chloride |
CaF2 | Calcium Fluoride |
C0 | Initial Fluoride Concentration (mg/L) |
Ce | Equilibrium (Residual) Fluoride Concentration (mg/L) |
CRediT | Contributor Roles Taxonomy |
FTIR | Fourier-transform Infrared Spectroscopy |
NaF | Sodium Fluoride |
pH | Potential of Hydrogen |
ppm | Parts per Million |
qe | Adsorption Capacity at Equilibrium (mg/g) |
R2 | Coefficient of Determination |
rpm | Revolutions per Minute |
SEM | Scanning Electron Microscopy |
WHO | World Health Organization |
°C | Degrees Celsius |
µm | Micrometre |
| [1] | World Health Organization. Guidelines for drinking-water quality: Fourth edition, incorporating the 1st addendum. Geneva: WHO; 2017 (reprinted with minor updates 2022). |
| [2] | Ayoob, S., & Gupta, A. K. (2006). Fluoride in drinking water: A review on the status and stress effects. Critical Reviews in Environmental Science and Technology, 36(6), 433-487. |
| [3] | Gizaw, M., et al. (2021). Fluoride in Ethiopian Rift groundwater: Occurrence and hydrogeochemical controls. Environmental Monitoring and Assessment, 193, 409. |
| [4] | Mutagaya, R., et al. (2019). Distribution of fluoride in groundwater of the Kenyan Rift Valley and implications for water supply. Science of the Total Environment, 659, 159-170. |
| [5] | Mkude, I. T., et al. (2022). Fluoride in Tanzanian waters: Occurrence, health risk and mitigation. Environmental Monitoring and Assessment, 194, 557. |
| [6] | Loganathan, P., Vigneswaran, S., Kandasamy, J., & Naidu, R. (2013). Removal of fluoride from water by adsorption—A review. Journal of Hazardous Materials, 248-249, 1-19. |
| [7] | Shen, J., & Schäfer, A. I. (2014). Removal of fluoride from water: A critical review of adsorption-based processes. Critical Reviews in Environmental Science and Technology, 44(22), 2455-2496. |
| [8] | Zhou, X., et al. (2023). A review on fluoride removal technologies for drinking water. RSC Advances, 13, 17267-17296. |
| [9] | Thakur, L. S., & Mondal, A. (2021). Low-cost biosorbents for fluoride removal: A review. Environmental Technology & Innovation, 22, 101427. |
| [10] | Ndabigengesere, A., Narasiah, K. S., & Talbot, B. G. (1995). Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Research, 29(2), 703-710. |
| [11] | Ghebremichael, K. A., Gunaratna, K. R., Henriksson, H., Brumer, H., & Dalhammar, G. (2005). A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Research, 39(11), 2338-2344. |
| [12] | Suneetha, J., & Syed Shafi, K. (2014). Removal of fluoride from water using powdered seeds of Moringa oleifera. International Journal of Engineering Research and Development, 10(7), 1-6. |
| [13] | Viswanathan, N., & Meenakshi, S. (2011). Removal of fluoride from aqueous solution using magnesium-incorporated biopolymer. Applied Water Science, 1, 17-24. |
| [14] | Wang, J., et al. (2022). Recent advances in composite adsorbents for fluoride removal from drinking water. Journal of Environmental Chemical Engineering, 10(4), 107543. |
| [15] | Zhang, Y., et al. (2021). Calcium-modified biochar for enhanced fluoride removal from water: Performance and mechanisms. Bioresource Technology, 319, 124199. |
| [16] | Fu, Y., et al. (2023). Adsorption isotherms, kinetics, and thermodynamics: A comprehensive review for water treatment. ACS Omega, 8(6), 5310-5330. |
| [17] | Ahmed, R., S. Patel, and G. Nair, Calcium-spiked Moringa oleifera seed adsorbent for enhanced fluoride removal from water. Journal of Water Process Engineering, 2023. 54: 104003. |
| [18] | Kumari, P., V. Singh, and R. Meena, Metal-ion modified Moringa biosorbents for defluoridation: Performance and mechanisms. Environmental Science and Pollution Research, 2022. 29(47): 71238-71252. |
| [19] | Mwangi, D., and L. Muthoni, Calcium-activated Moringa pods for defluoridation of Nakuru groundwater: Batch optimization and field appraisal. African Journal of Environmental Science and Technology, 2024. 18(2): 55-66. |
| [20] | Shrestha, B., P. Adhikari, and K. Sharma, Calcium-modified Moringa bark as a biosorbent for fluoride: Isotherms, kinetics and pH effects. Chemosphere, 2022. 303: 135108. |
| [21] | Nasreen, S., M. Rahman, and S. Akter, Comparative evaluation of zinc- and calcium-spiked agro-residues for fluoride removal from drinking water. Journal of Environmental Chemical Engineering, 2023. 11(6): 110245. |
| [22] | Asfaw, T., and A. Mekonnen, Calcium-treated cactus (Opuntia) biosorbent for defluoridation: Capacity enhancement and competitive ion effects. Bioresource Technology Reports, 2022. 19: 101195. |
| [23] | Elshazly, A., H. Abdel-Rahman, and N. F. Aly, Calcium-enhanced biochar composites for fluoride removal: Role of surface precipitation and pore filling. Separation and Purification Technology, 2023. 313: 123456. |
| [24] | Liu, Q., Y. Zhao, and J. Chen, Predictive adsorption modeling of calcium-functionalized biosorbents for fluoride removal. ACS Omega, 2025. 10(12): 12015-12026. |
| [25] | Moyo, S., T. Ncube, and P. Dube, Defluoridation of groundwater using Moringa oleifera seed powder: A review of performance and constraints. Water SA, 2016. 42(2): 256-263. |
| [26] | Aslam, Z., H. Khan, and M. Iqbal, Process water quality after defluoridation: pH shifts and post-treatment needs for alum, bone char and biosorbents. Desalination and Water Treatment, 2022. 252: 190-201. |
| [27] | Chavan, R. B., S. Kulkarni, and A. Patil, FTIR-SEM elucidation of calcium-functionalized Moringa oleifera seed powder for fluoride adsorption. Environmental Technology & Innovation, 2022. 28: 102834. |
| [28] | Feki, M., R. Djenizian, and A. Moussa, Fluoride removal using modified olive pomace: Capacity limits at high influent levels. Environmental Technology, 2023. 44(9): 1637-1649. |
APA Style
Chavaregi, G., Lusweti, J. K., Kipkemboi, P. K. (2025). Fluoride Removal Efficiency of Calcium-spiked and Non-spiked Moringa Oleifera Seed Powder. American Journal of Physical Chemistry, 14(4), 91-99. https://doi.org/10.11648/j.ajpc.20251404.11
ACS Style
Chavaregi, G.; Lusweti, J. K.; Kipkemboi, P. K. Fluoride Removal Efficiency of Calcium-spiked and Non-spiked Moringa Oleifera Seed Powder. Am. J. Phys. Chem. 2025, 14(4), 91-99. doi: 10.11648/j.ajpc.20251404.11
@article{10.11648/j.ajpc.20251404.11,
author = {Geoffrey Chavaregi and John Kituyi Lusweti and Pius Keronei Kipkemboi},
title = {Fluoride Removal Efficiency of Calcium-spiked and Non-spiked Moringa Oleifera Seed Powder
},
journal = {American Journal of Physical Chemistry},
volume = {14},
number = {4},
pages = {91-99},
doi = {10.11648/j.ajpc.20251404.11},
url = {https://doi.org/10.11648/j.ajpc.20251404.11},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.ajpc.20251404.11},
abstract = {Fluoride contamination in drinking water remains a widespread public health concern, particularly in arid and semi-arid regions where groundwater is the primary source of potable water. Chronic exposure to elevated fluoride levels—commonly above the World Health Organization’s (WHO) recommended limit of 1.5 mg/L—can result in dental and skeletal fluorosis, affecting millions of people globally. Affordable and effective defluoridation technologies are urgently needed, especially in low-income rural settings. In this study, the fluoride removal efficiency of calcium-spiked and non-spiked Moringa oleifera seed powder was investigated through controlled laboratory batch adsorption experiments. Biosorbents were prepared by treating ground seed powder with 1% calcium chloride solution and characterised based on their performance across five fluoride concentrations (1-20 ppm). Key parameters such as removal efficiency, residual fluoride levels, and adsorption capacity (qe) were evaluated under consistent operating conditions (pH 7, 2 g/50 mL dose, mesh 40, 120 minutes). Results indicated that calcium-spiked Moringa oleifera powder significantly outperformed its non-spiked counterpart. At 1 ppm, the spiked adsorbent achieved 94.35 ± 1.15% removal efficiency, compared to 81.45 ± 1.35% for the non-spiked. At the highest tested concentration (20 ppm), the spiked biosorbent still removed 72.31 ± 1.80% of fluoride, while the non-spiked removed only 54.21 ± 1.95%. Linear regression models showed strong inverse correlations between fluoride concentration and removal efficiency (R2 > 0.99, p p Moringa oleifera seed powder is a promising, low-cost, and environmentally friendly solution for mitigating fluoride contamination in drinking water.
},
year = {2025}
}
TY - JOUR T1 - Fluoride Removal Efficiency of Calcium-spiked and Non-spiked Moringa Oleifera Seed Powder AU - Geoffrey Chavaregi AU - John Kituyi Lusweti AU - Pius Keronei Kipkemboi Y1 - 2025/10/18 PY - 2025 N1 - https://doi.org/10.11648/j.ajpc.20251404.11 DO - 10.11648/j.ajpc.20251404.11 T2 - American Journal of Physical Chemistry JF - American Journal of Physical Chemistry JO - American Journal of Physical Chemistry SP - 91 EP - 99 PB - Science Publishing Group SN - 2327-2449 UR - https://doi.org/10.11648/j.ajpc.20251404.11 AB - Fluoride contamination in drinking water remains a widespread public health concern, particularly in arid and semi-arid regions where groundwater is the primary source of potable water. Chronic exposure to elevated fluoride levels—commonly above the World Health Organization’s (WHO) recommended limit of 1.5 mg/L—can result in dental and skeletal fluorosis, affecting millions of people globally. Affordable and effective defluoridation technologies are urgently needed, especially in low-income rural settings. In this study, the fluoride removal efficiency of calcium-spiked and non-spiked Moringa oleifera seed powder was investigated through controlled laboratory batch adsorption experiments. Biosorbents were prepared by treating ground seed powder with 1% calcium chloride solution and characterised based on their performance across five fluoride concentrations (1-20 ppm). Key parameters such as removal efficiency, residual fluoride levels, and adsorption capacity (qe) were evaluated under consistent operating conditions (pH 7, 2 g/50 mL dose, mesh 40, 120 minutes). Results indicated that calcium-spiked Moringa oleifera powder significantly outperformed its non-spiked counterpart. At 1 ppm, the spiked adsorbent achieved 94.35 ± 1.15% removal efficiency, compared to 81.45 ± 1.35% for the non-spiked. At the highest tested concentration (20 ppm), the spiked biosorbent still removed 72.31 ± 1.80% of fluoride, while the non-spiked removed only 54.21 ± 1.95%. Linear regression models showed strong inverse correlations between fluoride concentration and removal efficiency (R2 > 0.99, p p Moringa oleifera seed powder is a promising, low-cost, and environmentally friendly solution for mitigating fluoride contamination in drinking water. VL - 14 IS - 4 ER -